Landmark Opioid Lawsuit in Oklahoma Reveals More Pharmaceutical Culpability

In June 2019, in a courtroom in Norman, Oklahoma, lawyers for a huge pharmaceutical company are doing battle with lawyers representing Oklahomans who lost everything—even their lives—to opioid drugs. The defendant in this case is Johnson & Johnson. You’ve undoubtedly seen many of their products, maybe in your own home–products like BAND-AID® brand bandages, baby shampoo, and a popular mouthwash.
How could they end up in court defending themselves from claims that they helped cause this epidemic? It is because they are responsible for manufacturing and marketing two opioid products through their subsidiary, Janssen Pharmaceuticals: the extended-release opioid tablet Nucynta and a fentanyl patch called Duragesic.
In this lawsuit, lawyers for Oklahoma charge that through fraudulent marketing and kickbacks, Johnson & Johnson was right at the heart of creating a “public nuisance” that will cost between $12.6 billion and $17.5 billion to put right. Of course, that “public nuisance” also took the lives of thousands of Oklahomans.
Oklahoma isn’t alone in filing a lawsuit of this type. This is just one out of thousands of suits that have been filed against a long list of pharmaceutical manufacturers, marketers and distributors.
Why All the Lawsuits?
Because many people believe that the opioid epidemic that has killed hundreds of thousands of Americans didn’t “just happen.” They believe it was caused and that the beginning of this problem resides in the efforts of these wealthy companies to increase their profits, no matter what the cost to patients, consumers and the public.
Whatever the cause, the Centers for Disease Control and Prevention (CDC) informs us that between 1999 and 2017, more than 700,000 Americans have died from drug overdoses. Most years, two out of three of these deaths resulted from opioids.
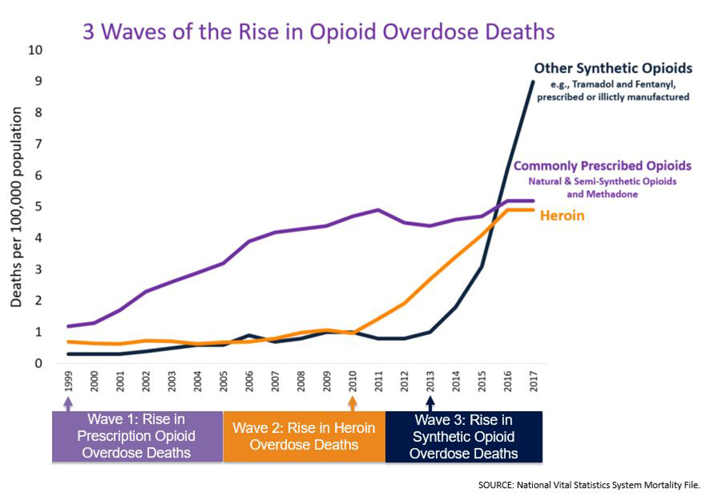
Here, you can see how the number of deaths has increased enormously in the last couple of decades. And according to the CDC, the first wave of these deaths were associated with a rise in the prescribing of prescription opioids.
Finally, two decades later, pharmaceutical companies are being forced to confront their misdeeds.
Are Pharmaceutical Companies Really to Blame?
If pharmaceutical companies marketed their products responsibly and ethically, they should have nothing to fear. But one after another, these large pharmaceutical corporations have been sued by states, counties, cities and other entities. Some of these suits were filed a couple of years ago and now, we’re starting to see out-of-court settlements resolving these suits.

- March 2019: In West Virginia, drug distributor McKesson Corp. agreed to pay the state $37 million for the company’s role in creating the state’s problems with opioids. The company admitted no wrongdoing but agreed to fund drug rehabilitation and mental health programs to cope with the fallout of addiction.
- West Virginia previously received settlements from other distributors such as Cardinal Health for $20 million and AmerisourceBergen for $16 million.
- March 2019: In Oklahoma, Purdue Pharma, the patent-holder for OxyContin, agreed to an out-of-court settlement of $270 million. Purdue still has more than 1,600 other lawsuits filed against them. Many people very familiar with the causes of the opioid epidemic, including journalist Sam Quinones, lay the origins of this overdose crisis at their door.
- May 2019: Teva Pharmaceuticals settled with Oklahoma for $85 million. They had been charged with downplaying the addictiveness of the opioid painkillers they manufacture and distribute. Teva distributes forms of fentanyl and oxycodone.
These suits against opioid-selling companies don’t constitute the first incidence of pharmaceutical companies being held accountable for their improprieties. In a case related to fraudulent marketing of the antipsychotic drug Risperdal, Johnson & Johnson agreed to a $2.2 BILLION settlement. The company was charged with misbranding this drug, encouraging doctors to prescribe it for symptoms such as anxiety or depression, especially among elderly dementia patients. The company also paid kickbacks to physicians who prescribed the drug.
Will Justice Prevail in Oklahoma?
For that, we will just have to wait and see. Chances are very good that Johnson & Johnson will agree to an out-of-court settlement if the evidence against them is strong enough.
As of today, the case is not looking good for Johnson & Johnson. On July 11, the state called the co-director of the Opioid Policy Research Collaborative at Brandeis University, Dr. Andrew Kolodny to testify. He stated that their misdeeds might even exceed those of Purdue Pharma’s. “Until I had an opportunity to review discovery documents, I really was not aware of how bad Johnson & Johnson was,” he said.
Oklahomans and Americans alike will have to follow this case to see if the families who lost loved ones to opioid prescriptions achieve a greater justice from this trial.
Reviewed by Claire Pinelli, ICAADC, CCS, LADC, RAS, MCAP


